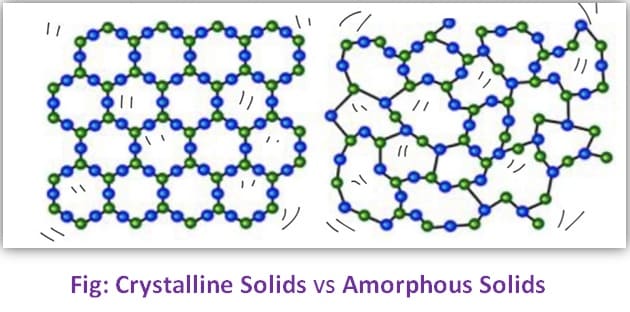
Crystalline Solids:
A Crystalline solid is a material in which the constituents are organized in a highly ordered microscopic structure. This structure forms a lattice, which extends in all directions. Crystalline solids have regular ordered arrays of components held together by uniform intermolecular forces.
Amorphous Solids:
The term “amorphous” refers to something that lacks a defined shape or structure. It is used to describe a substance or material that does not have a regular, repeating pattern of atoms or molecules, and therefore lacks a distinct crystalline structure.
Crystalline vs Amorphous Solids: