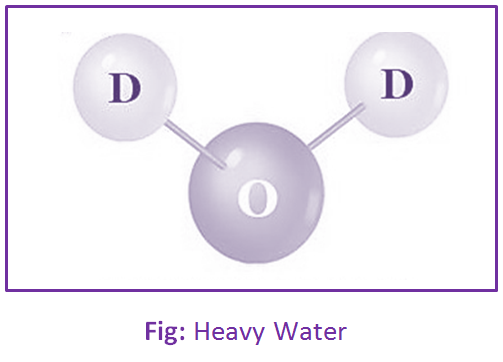
Hard Water:
Water that doesn’t easily produce lather with soap is called Hard Water. It contains a high concentration of calcium and magnesium ions. However, hardness can be caused by several other dissolved metals; those forms divalent or multi-valent cations, including aluminum, barium, strontium, iron, zinc, and manganese.
Example: Sea Water, Deep Tube Well Water, etc.
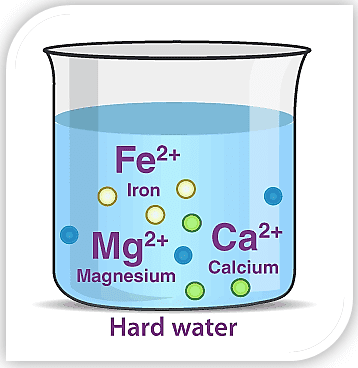
Heavy Water:
It is a form of water with a unique atomic structure and properties coveted for the production of nuclear power and weapons. Like ordinary water—H20 — each molecule of heavy water contains two hydrogen atoms and one oxygen atom.